Research
Role for delayed rectifier K channels (IK), and molecular mechanisms that underlie arrhythmias acquired in obesity
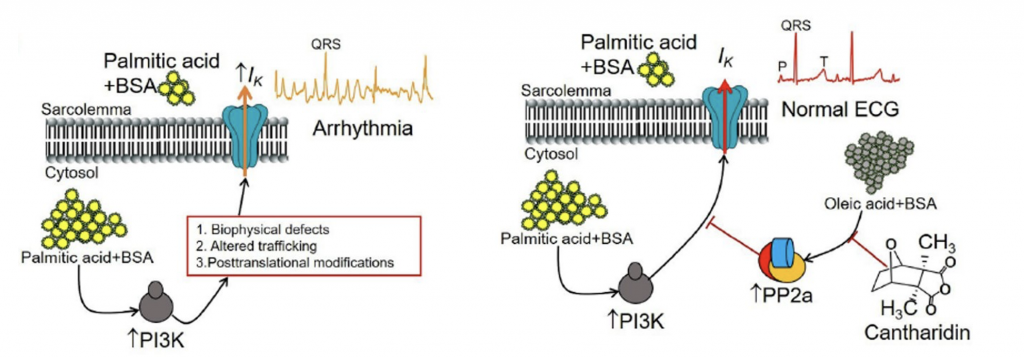
Molecular mechanisms linking dietary interventions that include the saturated free-fatty acid (FFA) palmitic acid and the monounsaturated oleic acid activated pathways to atrial electrical remodeling. Our preliminary findings demonstrate a role for PI3K as a key intermediate mediator linking obesity pathological mechanisms to supraventricular arrhythmias.
Electrical properties of guinea pig ex vivo hearts
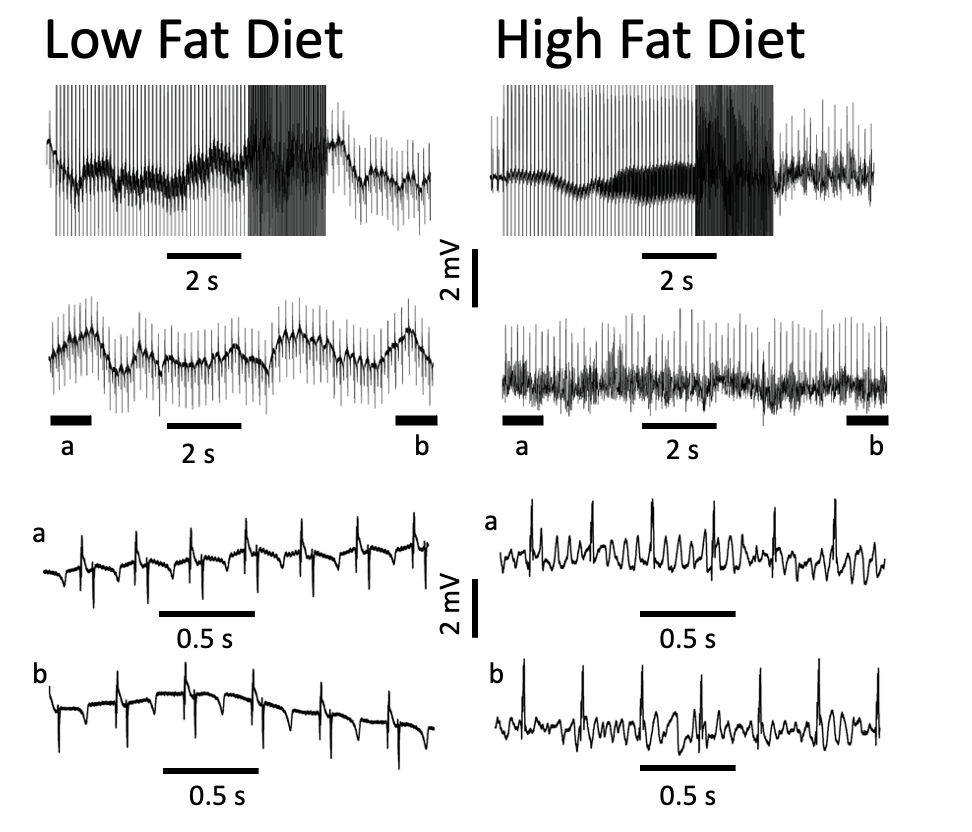
Induction of arrhythmogenesis in right atria of isolated Langendorff perfused hearts from LFD- (left panel) and HFD-fed (right panel) guinea pigs. With rapid right atrial pacing (burst, rectangular pulses 10 ms, 1 V in amplitude with pulse-pulse duration ranging between 20 and 250 ms). Expanded view of atria electrical activities revealed sinus rhythm with LFD (left panel, bottom traces) and uncoordinated atrial activities in HFD-fed guinea pigs (right panel, bottom traces) consistent with AF. Adaptedfrom Martinez-Mateu L, et al Front Physiol (2019).
Functional interplay between cardiac macrophages and myocytes
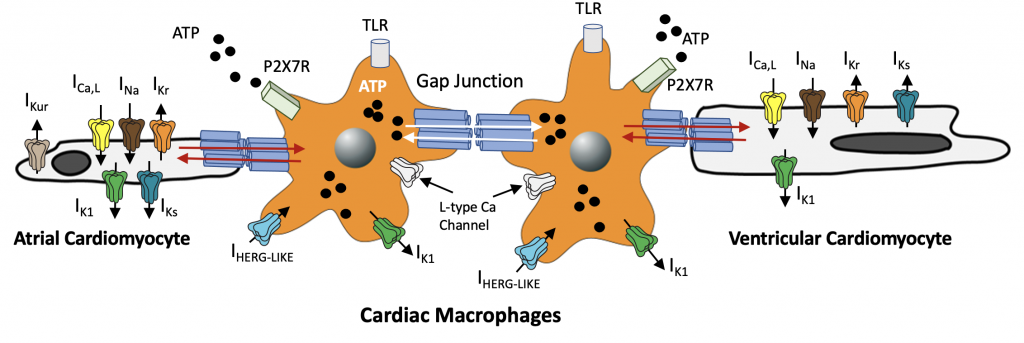
Macrophages express ion channels that are similar to depolarizing (ICa, INa) and repolarizing mechanisms in heart (IKr, IKs, and IK1). What role, if any do the expression of these channels play in mediating the effects of free-fatty acids on cardiac electrical activity? Despite the lack of clarity, macrophages are likely to influence cardiac electrical function to help maintain homeostatic control. Comprehensive investigation of this functional interaction is likely to improve our current understanding of the role of cardiac macrophages in inflammation and the pathogenesis of lipotoxic cardiomyopathy. Alí A, et al Front. Physiol. 9 (2018).
Spatial location of hERG subunits in guinea pig ventricular myocytes
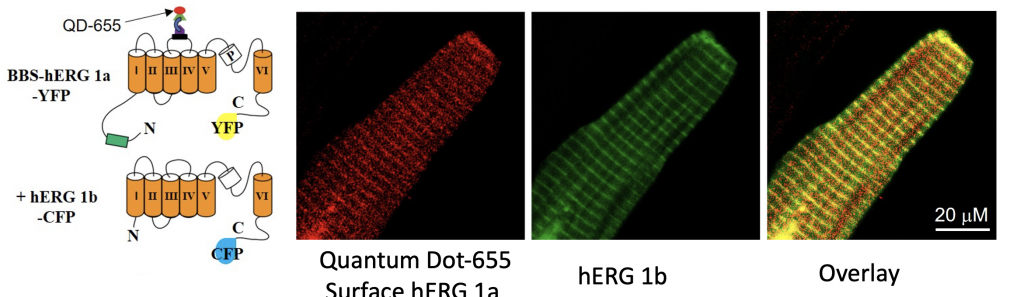
Colocalization of virally expressed BBS- and YFP tagged hERG 1a and hERG 1b-CFP at the surface sarcolemma, t-tubule and intercalated disk. Cartoon illustration Adaptedfrom Martinez-Mateu L, et al Front Physiol (2019). The spatiotemporal and biophysical properties of individual ion channels allow the exchange of ions between distinct compartments, and are responsible for generation of an action potential in myocytes. Efficient cellular trafficking, surface expression and function of Na, Ca and K channels is vital for normal electrical activity because pathological changes in the relative functional properties and/or expression of cardiac ion channels is likely to disrupt the fine balance between these currents, with significant implications for cardiac repolarization and ultimately the transition to arrhythmias.